Multiple Choice
Determine the amount of energy released in this decay. Use the following atomic masses: 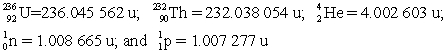 Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 J
Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 J
A) 3.5 × 10-8 J
B) 6.0 × 10-10 J
C) 4.6 × 10-12 J
D) 7.3 × 10-13 J
E) 2.9 × 10-12 J
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which process is involved in determining the
Q3: Which one of the following descriptive terms
Q4: The half-life a particular isotope of barium
Q5: Tritium is an isotope of hydrogen
Q6: Which of the following is not an
Q8: What is the SI unit for activity?<br>A)Ci<br>B)counts/min<br>C)Hz<br>D)Gy<br>E)Bq
Q9: The activity of carbon-14 in a sample
Q10: The half-life of a particular isotope of
Q11: What is X?<br>A) <span class="ql-formula" data-value="\alpha"><span
Q12: This question refers to the figure shown.