Multiple Choice
An electron in a hydrogen atom is described by the quantum numbers: n = 7 and ml = 4. What are the possible values for the orbital quantum number 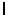 ?
?
A) only 0 or 4
B) only 4 or 7
C) only 5 or 6
D) only 4, 5, or 6
E) only 5, 6, or 7
Correct Answer:

Verified
Correct Answer:
Verified
Q34: Determine the wavelength of incident electromagnetic radiation
Q35: The principle quantum number for the electron
Q36: Which one of the following statements best
Q37: Which one of the following statements is
Q38: Name the physicist credited with the following
Q40: The figure shows an energy level diagram
Q41: Which one of the following statements concerning
Q42: Which electron energy will produce the largest
Q43: A neutral atom has the following electronic
Q44: How many electrons could be accommodated in