Multiple Choice
Which one of the following sets of quantum numbers is not possible? 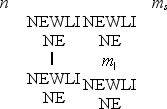
A) 
B) 
C) 
D) 
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: How many electrons could be accommodated in
Q45: The nucleus of a certain atom has
Q46: Complete the following statement: In the laser-based
Q47: Determine the energy of the photon emitted
Q48: Which one of the following subshells is
Q50: Consider the following list of electron configurations:
Q51: Which one of the following pairs of
Q52: An electron is in the ground state
Q53: To which model of atomic structure does
Q54: Which one of the following statements concerning