Multiple Choice
An electron in an atom has the following set of quantum numbers:
n = 2, 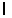 = 1,
= 1, 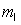 = -1, ms = +1/2.
= -1, ms = +1/2.
-What shell is this electron occupying?
A) K shell
B) L shell
C) M shell
D) N shell
E) O shell
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: Each atom in the periodic table has
Q29: An electron makes a transition from a
Q30: Each atom in the periodic table has
Q31: According to the Bohr model, what is
Q32: Complete the following sentence: Holography is<br>A)the projection
Q34: Determine the wavelength of incident electromagnetic radiation
Q35: The principle quantum number for the electron
Q36: Which one of the following statements best
Q37: Which one of the following statements is
Q38: Name the physicist credited with the following