Multiple Choice
The figure shows an energy level diagram for the hydrogen atom. Several transitions are shown and are labeled by letters. 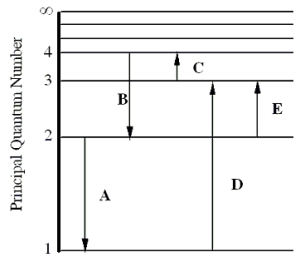 Note: The diagram is not drawn to scale.
Note: The diagram is not drawn to scale.
-Determine the energy of the photon involved in transition E.
A) 1.5 eV
B) 1.9 eV
C) 3.4 eV
D) 10.2 eV
E) 12.1 eV
Correct Answer:

Verified
Correct Answer:
Verified
Q59: Electrons in an X-ray tube are accelerated
Q60: The kinetic energy of the ground state
Q61: An argon-ion laser emits a blue-green beam
Q62: The second ionization energy (the energy required
Q63: Which quantum number applies to most of
Q65: What energy (in eV) is required to
Q66: A neutral atom has the following electronic
Q67: Consider the following list of electron configurations:
Q68: According to the quantum mechanical picture of
Q69: The figure shows an energy level diagram