Multiple Choice
A physicist wishes to produce electrons by shining light on a metal surface. The light source emits light with a wavelength of 450 nm. The table lists the only available metals and their work functions. 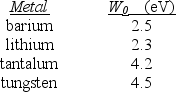
-Which entry in the table below correctly identifies the metal that will produce the most energetic electrons and their energies? 
A) 
B) 
C) 
D) 
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Q16: The x component of the velocity of
Q18: A proton (m<sub>p</sub> = 1.673 ×
Q19: When ultraviolet photons with a wavelength of
Q20: The position of a hydrogen atom (m
Q23: What is the speed of an electron
Q25: If Planck's constant were changed to 660
Q26: In a computer monitor, electrons approach the
Q27: Electrons are emitted from a certain metal
Q40: A physicist wishes to produce electrons by
Q42: Which one of the following quantities is