Multiple Choice
Beneath the moveable piston in the drawing,2.25 moles of a monatomic ideal gas is enclosed at 314 K. The initial volume of the gas is 3.0 m3. The gas is compressed isothermally to a final volume of 1.0 m3. How much heat is removed from the gas during this process? 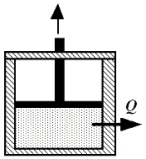
A) -6450 J
B) -4300 J
C) -2900 J
D) -1450 J
E) zero joules
Correct Answer:

Verified
Correct Answer:
Verified
Q6: A fixed amount of ideal gas is
Q27: An ideal monatomic gas expands isothermally from
Q34: A heat engine operates between a hot
Q57: A heat engine operates between a hot
Q68: A Carnot engine has a heat input
Q70: Under which one of the following conditions
Q74: A heat engine operates between a hot
Q75: An ideal gas is taken from state
Q77: Two engines are identical except that engine
Q78: What are the SI units of the