Multiple Choice
A 200.0-kg object is attached via an ideal pulley system to paddle wheels that are submerged in 0.480 kg of glycerin at 20.0 °C in an insulated container as shown. Then, the object falls through a distance of 5.00 m causing the paddle wheel to turn. Assuming all of the mechanical energy lost by the falling object goes into the water, determine the final temperature of the glycerin. The specific heat capacity of glycerin is 2410 J/ 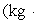 Co) .
Co) . 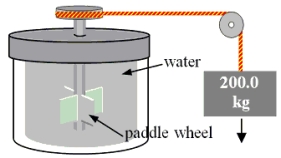
A) 4.90 °C
B) 28.5 °C
C) 24.9 °C
D) 40.4 °C
E) 8.47 °C
Correct Answer:

Verified
Correct Answer:
Verified
Q56: In an insulated container, 0.50 kg of
Q57: Given the following information, determine the relative
Q58: A 0.0500-kg lead bullet of volume 5.00
Q59: At a certain temperature, a simple pendulum
Q60: Three thermometers are in the same water
Q61: A soft drink manufacturer claims that a
Q63: Complete the following statement: When a substance
Q64: The coefficient of linear expansion of steel
Q65: Ryan places 0.150 kg of boiling water
Q66: A 2.00-kg metal block slides on a