Multiple Choice
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 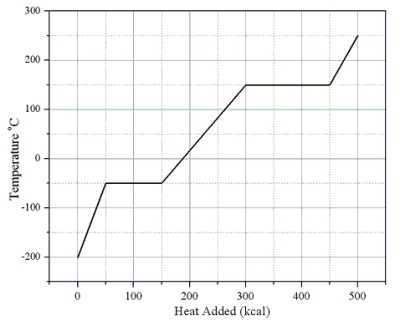
-What is the latent heat of fusion of this material?
A) 50 cal/g
B) 100 cal/g
C) 150 cal/g
D) 300 cal/g
E) 450 cal/g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: Heat is added to a 1.0-kg solid
Q18: Three thermometers are placed in a closed,
Q19: Which would cause a more serious burn:
Q20: The specific heat capacity of iron is
Q21: Two spheres, labeled A and B, have
Q23: A tanker ship is filled with 2.25
Q24: A 0.0500-kg lead bullet of volume 5.00
Q25: Zirconium tungstate is an unusual material
Q26: A 0.30-kg lead ball is heated to
Q27: Complete the following statement: When solid NH<sub>3</sub>