Multiple Choice
Consider the following van der Waals coefficients: 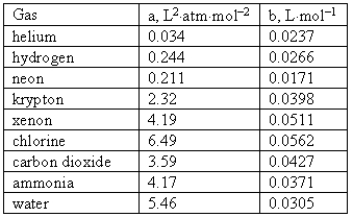 Which of the following gases has the largest attractive forces?
Which of the following gases has the largest attractive forces?
A) Neon
B) Ammonia
C) Chlorine
D) Water
E) Helium
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Sulfur dioxide reacts with oxygen gas
Q28: The pressure at 20,000 feet above sea
Q58: A sample of nitrogen gas collected
Q67: The value of the gas law constant,R,in
Q106: Which molecules of the following gases will
Q107: At 80.0<sup> <span class="ql-formula" data-value="\omicron "><span
Q130: If the average speed of a
Q148: The following experiment was carried out using
Q234: How many atoms of helium occupy 100.0
Q260: Sulfur dioxide reacts with oxygen gas