Multiple Choice
Consider the following van der Waals coefficients: 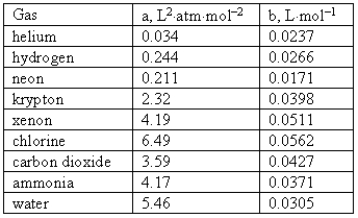 Which of the following gases has the smallest attractive forces?
Which of the following gases has the smallest attractive forces?
A) Ammonia
B) Hydrogen
C) Neon
D) Helium
E) Chlorine
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: A Group 17 or Group 18
Q15: What volume is occupied by 1,000.g of
Q36: What is the density of nitrogen
Q39: All of the following elements are gases
Q39: A 1.00-L sample of C<sub>2</sub>H<sub>4</sub>(g)at 2.00 atm
Q76: If it takes 15 s for a
Q103: A sample of gas with a
Q134: What mass of zinc metal is required
Q202: Which gas is least dense at
Q262: If the average speed of a