Multiple Choice
What is the formula of the superconductor whose unit cell is shown below? (The Y and Ba atoms are in the middle of the cell.) 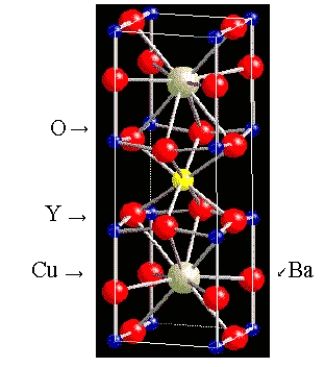
A) YBa2Cu3O9
B) YBa2Cu4O5
C) YBa2Cu2O7
D) YBa2Cu4O7
E) YBa2Cu3O7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: If the radius of an atom is
Q54: Viscosity usually decreases with increasing temperature.
Q70: What are the coordination numbers of Ca<sup>2+</sup>
Q84: Which of the following compounds is likely
Q124: An amorphous solid is one in which
Q149: If the ratio of the radius of
Q188: When the cations and anions of an
Q205: The boiling points of the Group 14
Q252: Gold has a face-centered cubic unit cell.If
Q266: Which has the higher boiling point,1,1-dichloroethene or