Multiple Choice
The structure of Tylenol is given below: 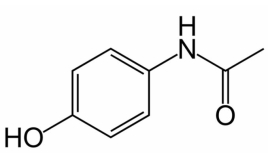 What hybrid orbitals are used on the N atom and the carbonyl carbon,
What hybrid orbitals are used on the N atom and the carbonyl carbon,
Respectively?
A) sp3 and sp2
B) sp2 and sp2
C) sp3 and sp3
D) sp2 and sp
E) sp3 and sp
Correct Answer:

Verified
Correct Answer:
Verified
Q132: Why are the N-O bond lengths in
Q133: In the NO molecule,which atom makes the
Q134: Which of the following species are radicals?<br>A)
Q135: Which of the following is polar?<br>A) CO<sub>3</sub><sup>2</sup><sup>-</sup><br>B)
Q136: Which of the following compounds is the
Q138: Which of the following is diamagnetic?<br>A) O<sub>2</sub><sup>2</sup><sup>-</sup><br>B)
Q139: White phosphorus is composed of tetrahedral molecules
Q140: All the following have an angular shape
Q141: Estimate the CO bond length in acetone,
Q142: Use the bond enthalpies given to estimate