Multiple Choice
What is the formula of the superconductor whose unit cell is shown below?
(The Y and Ba atoms are in the middle of the cell.) 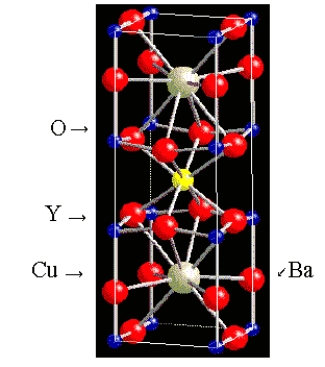
A) YBa2Cu3O9
B) YBa2Cu4O5
C) YBa2Cu2O7
D) YBa2Cu4O7
E) YBa2Cu3O7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q97: Predict which of the following liquids has
Q98: A 0.479-g sample of nitrogen, oxygen
Q99: What is graphene?<br>A) A three dimensional layer
Q100: Which of the following has the lowest
Q101: Upon what are silicates based?<br>A) Repeating silicon
Q103: A sample of gas with a
Q104: How many octahedral holes are there in
Q105: What is the density of chlorine
Q106: Which molecules of the following gases will
Q107: At 80.0<sup> <span class="ql-formula" data-value="\omicron "><span