Short Answer
For the reaction 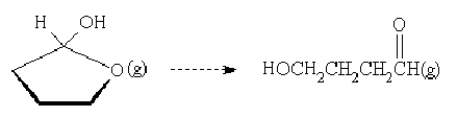
is the entropy change positive or negative?
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: Calculate the standard enthalpy of vaporization of
Q35: If the standard enthalpy of combustion of
Q36: Consider the compounds<br>PCl<sub>5</sub>(g),HCN(g),CuO(s),NO(g),NH<sub>3</sub>(g),And SO<sub>2</sub>(g).<br>Which compound will become
Q37: An isolated system can exchange energy and
Q38: When calculating the entropy of vaporization
Q40: Use the following information to determine
Q41: The standard enthalpy of formation of
Q42: The energy levels of two particle in
Q43: For any isothermal process, <span
Q44: Calculate the change in molar entropy when