Short Answer
The energy levels of two particle in a box systems are 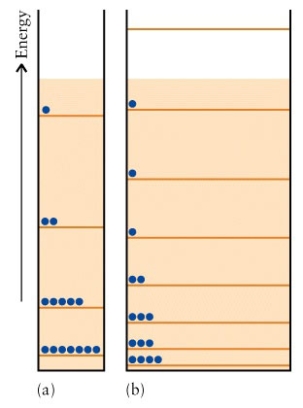 Which system has the higher entropy?
Which system has the higher entropy?
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q57: Use Trouton's constant to estimate the enthalpy
Q58: Use tabulated thermodynamic data to estimate the
Q59: Iodine sublimes at room temperature .The reaction
Q60: Sketch a plot of the molar entropy
Q61: The molar entropy of silver at 298
Q63: Calculate the normal boiling point of chloroform,
Q64: Given the standard reaction enthalpies below:<br>N<sub>2</sub>(g)+
Q65: When a gas expands into a vacuum,w
Q66: The hydrolysis of ATP to ADP is
Q67: The combustion of 1 mole of ethanol,