Multiple Choice
The phase diagram for a pure substance is shown below. 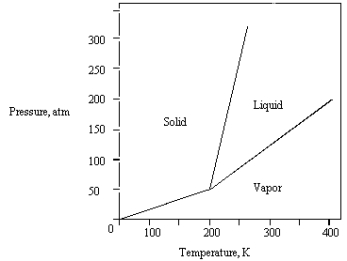 At 100 atm and 250 K,
At 100 atm and 250 K,
The substance exists as
A) both vapor and liquid in equilibrium.
B) liquid.
C) both vapor and solid in equilibrium.
D) vapor.
E) solid.
Correct Answer:

Verified
Correct Answer:
Verified
Q117: Given:<br>4NH<sub>3</sub>(g)+ 5O<sub>2</sub>(g) <span class="ql-formula" data-value="f"><span
Q118: The phase diagram for CO<sub>2</sub> is given
Q119: A plot of ln(vapor pressure) versus 1/T
Q120: Consider the reaction<br>N<sub>2</sub>(g)3H<sub>2</sub>(g) <span class="ql-formula"
Q121: Consider the reaction<br>PCl<sub>5</sub>(g) <span class="ql-formula"
Q123: the triple point for water,4.6
Q124: Consider the following reaction at a
Q125: Which of the following reactions is
Q126: An animal cell assumes its normal volume
Q127: Given:<br>SO<sub>2</sub>(g) <br> <span class="ql-formula" data-value="f"><span class="katex"><span