Multiple Choice
The phase diagram for a pure substance is given below. 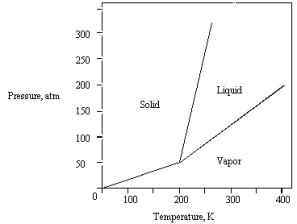 The substance is stored in a container at 150 atm at 25 C.Describe what happens if the container is opened at 25 C.
The substance is stored in a container at 150 atm at 25 C.Describe what happens if the container is opened at 25 C.
A) The liquid in the container freezes.
B) The solid in the container sublimes.
C) The solid in the container melts.
D) The vapor in the container escapes.
E) The liquid in the container vaporizes.
Correct Answer:

Verified
Correct Answer:
Verified
Q152: Consider the reaction<br>2CuBr<sub>2</sub>(s) <span class="ql-formula" data-value="\rightarrow"><span
Q153: Consider the following reaction at a
Q154: For the reaction<br>NH<sub>3</sub>(g)+ H<sub>2</sub>S(g) <span
Q155: Which of the following 1.0 M solutions
Q156: For AgI, the lattice enthalpy is
Q158: Given: 2SO<sub>2</sub>(g) + O<sub>2</sub>(g) <span
Q159: What does a negative slope for the
Q160: The phase diagram for a pure substance
Q161: For the reaction<br>N<sub>2</sub>O<sub>4</sub>(g) <span class="ql-formula"
Q162: For a pure solid or liquid,the molar