Multiple Choice
The phase diagram for CO2 is given below .
The triple point is at 5.1 atm and 217 K. 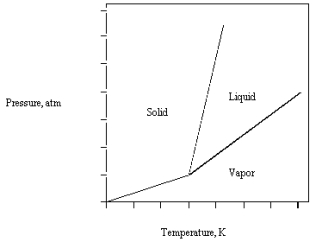
What happens if carbon dioxide at 50C and 25 atm is suddenly brought to 1 atm?
A) The liquid and solid are in equilibrium.
B) The solid melts.
C) The solid and vapor are in equilibrium.
D) The solid vaporizes.
E) The solid remains stable.
Correct Answer:

Verified
Correct Answer:
Verified
Q62: Given: 2SO<sub>2</sub>(g) + O<sub>2</sub>(g) <span
Q63: If the equilibrium constant for the
Q64: The equilibrium constant, K,For the reaction<br>2HgO(s)
Q65: Calculate the number of moles of
Q66: Which of the following liquids freeze at
Q68: Calculate the vapor pressure of ethyl alcohol,
Q69: Blood, sweat,And tears are about 0.15
Q70: For CaCl<sub>2</sub>, the absolute value of the
Q71: For the reaction<br>2CaSO<sub>4</sub>(s)<br> <span class="ql-formula" data-value="f"><span
Q72: the van't Hoff i of HBr,HCl,and HF