Essay
Consider the diagram below. 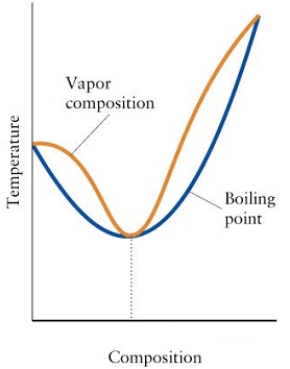
(a)What is the mixture in the diagram called?
(b)Can the components of this mixture be separated by fractional distillation? Explain.
Correct Answer:

Verified
(a)Minimum-boiling azeotrope
(...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
(...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q50: For the reaction 2NOCl(g) <span
Q51: A mixture consisting of 0.250 M
Q52: The phase diagram for sulfur is given
Q53: Consider the reaction<br>PCl<sub>5</sub>(g) <span class="ql-formula"
Q54: For AlF<sub>3</sub>,the lattice enthalpy is 5220
Q56: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math
Q57: Consider the following reaction at a
Q58: For the reaction Br<sub>2</sub>(g)<br> <span
Q59: At 600<font face="symbol">?</font>C, the equilibrium constant
Q60: The phase diagram for a pure substance