For the Equilibrium CaCO3(s) CaO(s)+ CO2(g),
(A)represents the Composition at Equilibrium at a Certain
Essay
For the equilibrium CaCO3(s) CaO(s)+ CO2(g),
(a)represents the composition at equilibrium at a certain temperature.In (b),a small amount of Ca*CO3(s) has been added (Ca*CO3(s)represents Ca14CO3(s)or labeled calcium carbonate).Draw the composition in (c) at equilibrium and explain your drawing. 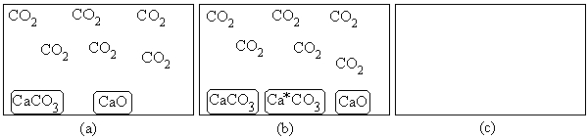
Correct Answer:

Verified
CaCO3,Ca*CO3,CaO,and a ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q70: For CaCl<sub>2</sub>, the absolute value of the
Q71: For the reaction<br>2CaSO<sub>4</sub>(s)<br> <span class="ql-formula" data-value="f"><span
Q72: the van't Hoff i of HBr,HCl,and HF
Q73: Which of the following has the lowest
Q74: If <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q76: At 25<font face="symbol">?</font>C, K<sub> </sub> =
Q77: Consider the reaction<br>2SO<sub>2</sub>(g)+O<sub>2</sub>(g) <span class="ql-formula" data-value="\rightarrow"><span
Q78: The lattice enthalpy and the enthalpy of
Q79: What is the vapor pressure of carbon
Q80: The equilibrium constant expression for the