Short Answer
The curve for the titration of 50.0 mL of 0.0200 M C6H5COOH(aq)
with 0.100 M NaOH(aq) is given below.What are the main species in the solution after 7.5 mL of base have been added? 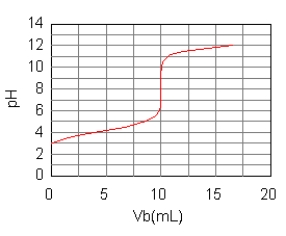
Correct Answer:

Verified
C6H5...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q104: The standard voltage of the cell<br>Pt|H<sub>2</sub>(g)|H<sup>+</sup>(aq)M
Q105: Consider the following cell:<br>Zn(s)|Zn<sup>2+</sup>(aq,0.10 M)m Cu<sup>2+</sup>(aq,0.10 M)|Cu(s)<br>At
Q106: In a working electrochemical cell (+ cell
Q107: Which of the following produces the strongest
Q108: If 50.0 mL of 0.22 M NaCl(aq)is
Q110: If the value of K<sub>b</sub> for
Q111: Both H<sub>2</sub>O and OH<sup>-</sup>can act as a
Q112: Which of the following is the weakest
Q113: The half-reaction that occurs at cathode when
Q114: Which of the following is the strongest