Multiple Choice
The titration curve for the titration of 0.100 M H2SO3(aq) with 0.100 M KOH(aq) Is given below. 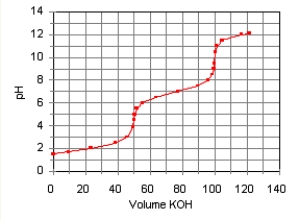
The major species in solution after 75 mL of KOH(aq) Has been added are
A) HSO3-(aq) and Na+(aq) .
B) SO32-(aq) , and Na+(aq) .
C) SO32-(aq) , OH-(aq) , and Na+(aq) .
D) H2SO3(aq) , HSO3-, and Na+(aq) .
E) HSO3-(aq) , SO32-(aq) , and Na+(aq) .
Correct Answer:

Verified
Correct Answer:
Verified
Q187: Use the following to answer questions 55-58:
Q188: What is the [H<sup>+</sup>] for a solution
Q189: The following compounds are available as
Q190: Given: Zn(s) + OH<sup>-</sup>(aq)+ H<sub>2</sub>O(l)+ NO<sub>3</sub><sup>-</sup>(aq
Q191: The weak base, B,Has K<sub>b</sub> = 3.1
Q193: Which of the following mixtures gives a
Q194: In the determination of iron in vitamins,
Q195: Assuming no volume change on mixing,what mass
Q196: The pH of 0.010 M H<sub>3</sub>PO<sub>4</sub>(aq) is
Q197: What is the pH of an aqueous