Multiple Choice
The titration curve for the titration of 0.100 M Na2CO3(aq) with 0.100 M HClO4(aq)
Is: 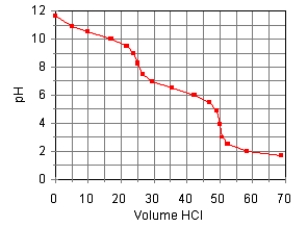
Estimate pKb1.
A) 8.5
B) 6.4
C) 3.7
D) 10.3
E) 7.6
Correct Answer:

Verified
Correct Answer:
Verified
Q88: Consider the following cell:<br>Zn(s)|Zn<sup>2+</sup>(aq,0.200 M)M H<sup>+</sup>(aq,?)|H<sub>2</sub>(g,1.00
Q89: What is the molarity of OH<sup>-</sup> in
Q90: Which species will reduce Ag<sup>+</sup> but not
Q91: Which of the following water-insoluble salts is
Q92: Which pair of metals will dissolve in
Q94: Which of the following occurs when HNO<sub>3</sub>(aq),
Q95: If the pK<sub>a</sub> of acetic acid is
Q96: How many moles of KOH(s)must be added
Q97: The amino acid methionine, HOOC-CH(CH<sub>2</sub>CH<sub>2</sub>SCH<sub>3</sub>)NH<sub>3</sub><sup>+</sup>,Has pK<sub>a1</sub> =
Q98: What is the conjugate base of H<sub>2</sub>PO<sub>4</sub><sup>-</sup>