Multiple Choice
The titration curve for the titration of 0.100 M Na2CO3(aq) with 0.100 M HClO4(aq)
Is: 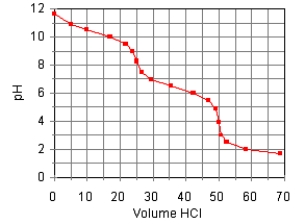
Estimate pKb2.
A) 7.6
B) 10.3
C) 6.4
D) 8.5
E) 3.7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q64: The pH of a 0.0050 M aqueous
Q65: Calculate the equilibrium constant for the reaction
Q66: Which species will reduce Br<sub>2</sub> but not
Q67: When CaO(s) is dissolved in water,Which of
Q68: The fractional composition diagram for the amino
Q70: What is the pH at the stoichiometric
Q71: The following compounds are available as
Q72: Given: S<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup>(aq) <span class="ql-formula" data-value="\rightarrow"><span
Q73: Calculate the value of the equilibrium
Q74: If 10.0 mmol of sodium hydroxide is