Essay
Use the following to answer questions 55-58: 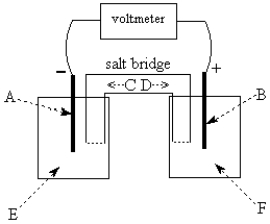
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
Correct Answer:

Verified
A IS Zn(S); Zn(S) Zn...
Zn...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q262: Choose the effective pH range of a
Q263: Which metal will dissolve in hydrochloric acid?<br>A)
Q264: What is E for the half-reaction
Q265: Consider the following cell:<br>Pt|H<sub>2</sub>(g,1 atm)|H<sup>+</sup>(aq,? M)M
Q266: Which of the following indicators would be
Q268: Which of the following is the conjugate
Q269: The following 0.1 M aqueous solutions
Q270: Write the charge balance equation for a
Q271: Of the following, which is the strongest
Q272: When the Ag(s)|AgCl(s|Cl<sup>-</sup>(aq) electrode acts as