True/False
Use the following to answer questions 55-58: 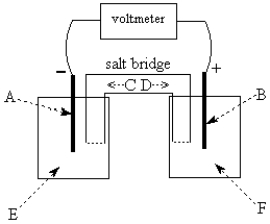
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Theelectrode B could be inert platinum metal or lead.
Correct Answer:

Verified
Correct Answer:
Verified
Q272: When the Ag(s)|AgCl(s|Cl<sup>-</sup>(aq) electrode acts as
Q273: The solubility of silver bromide is greater
Q274: If a small amount of a strong
Q275: The K<sub>a</sub> of phenol is 1.3 *
Q276: A buffer solution contains 0.75 mol KH<sub>2</sub>PO<sub>4</sub>
Q278: What is the pH of an aqueous
Q279: If 8686 C of charge is passed
Q280: If pK<sub>a1</sub> and pK<sub>a2</sub> for H<sub>2</sub>S
Q281: Consider the following cell:<br>Pt|Fe<sup>2+</sup>(aq,0.50 M),Fe<sup>3+</sup>(aq,0.30 M)m
Q282: Given:<br>Ag<sup>+</sup>(aq)+ e<sup>-</sup> <span class="ql-formula" data-value="\rightarrow"><span