True/False
Use the following to answer questions 55-58: 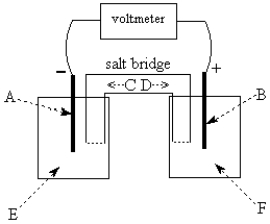
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or zinc.
Correct Answer:

Verified
Correct Answer:
Verified
Q157: For a solution of phosphoric acid, write
Q158: What is the pH of an aqueous
Q159: When pyridinium chloride is added to C<sub>5</sub>H<sub>5</sub>N(aq),<br>A)
Q160: Which of the following indicators would be
Q161: What is the main factor that directly
Q163: Choose the effective pH range of an
Q164: Write the autoprotolysis reaction for liquid ammonia.
Q165: If pK<sub>a1</sub> and pK<sub>a2</sub> for H<sub>2</sub>CO<sub>3</sub>
Q166: If a small amount of a strong
Q167: What is the pH of an aqueous