Multiple Choice
Use the following diagram of a cell to answer questions 59-64: 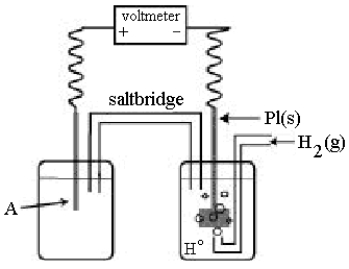
-In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE) .If the voltmeter reading is -0.76 V,
Which half-reaction occurs in the left-hand cell compartment?
A) Zn2+(aq) + 2e- Zn(s)
B) Zn(s) Zn2+(aq) + 2e-
Correct Answer:

Verified
Correct Answer:
Verified
Q163: Choose the effective pH range of an
Q164: Write the autoprotolysis reaction for liquid ammonia.
Q165: If pK<sub>a1</sub> and pK<sub>a2</sub> for H<sub>2</sub>CO<sub>3</sub>
Q166: If a small amount of a strong
Q167: What is the pH of an aqueous
Q169: In the Daniell cell,the anode is zinc
Q170: Consider the following cell at standard
Q171: If the standard potential for Cu<sup>2+</sup>(aq)/Cu<sup>+</sup>(aq)
Q172: A buffer solution contains 0.0200 M acetic
Q173: If the molar solubility of the compound