Multiple Choice
Use the following diagram of a cell to answer questions 59-64: 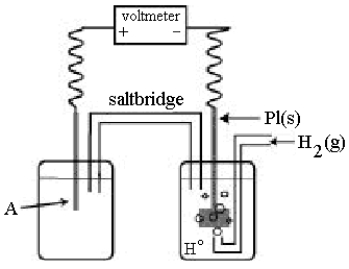
-In the cell shown above, A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE) .If the voltmeter reading is +0.80 V,
What is the equation for the cell reaction?
A) Ag(s) + H+(aq) Ag+(aq) + ½H2(g)
B) Ag+(aq) + ½H2(g) Ag(s) + H+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q173: If the molar solubility of the compound
Q174: Which of the following aqueous solutions gives
Q175: The pH of 0.010 M aniline(aq) is
Q176: How many seconds are required to produce
Q177: For the following acids, which has the
Q179: Consider the titration of 50.0 mL of
Q180: Use the following diagram of a
Q181: Which of the following is the weakest
Q182: Consider the titration of 15.0 mL of
Q183: Of the following metals, which metal would