Short Answer
Use the following diagram of a cell to answer questions 59-64: 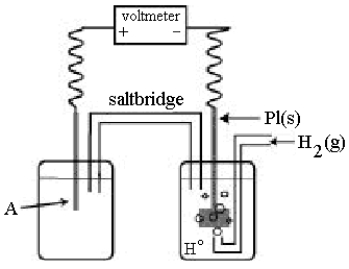
-In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,
Which electrode is negative?
Correct Answer:

Verified
Correct Answer:
Verified
Q26: For the titration of 50.0 mL of
Q27: Given: N<sub>2</sub>H<sub>5</sub><sup>+</sup>(aq) <span class="ql-formula" data-value="\rarr"><span
Q28: The following compounds are available as
Q29: What is the proper cell diagram
Q30: Calculate the [H<sup>+</sup>] in an aqueous solution
Q32: What is the pH at the half-stoichiometric
Q33: You have available the following reagents as
Q34: A certain weak acid has a K<sub>a</sub>
Q35: A 0.0010 M solution of a weak
Q36: Calculate the equilibrium constant for the reaction