Short Answer
Use the following diagram of a cell to answer questions 59-64: 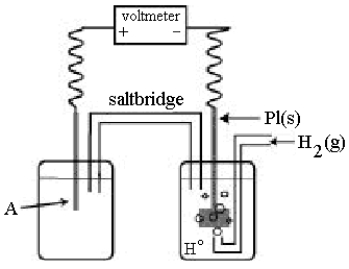
-In the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,which electrode is negative?
Correct Answer:

Verified
Correct Answer:
Verified
Q51: Calculate the hydroxide ion concentration for an
Q52: Calculate the equilibrium constant for the reaction
Q53: Bond polarity tends to dominate the trend
Q54: How many moles of O<sub>2</sub>(g) are produced
Q55: What is the pH at the stoichiometric
Q57: The amino acid alanine,HOOC-CH(CH<sub>3</sub>)NH<sub>3</sub><sup>+</sup>,Has K<sub>a1</sub> =
Q58: At the stoichiometric point in the titration
Q59: The following 0.1 M aqueous solutions
Q60: If the standard potential for Ti<sup>3+</sup>(aq)/Ti<sup>2+</sup>(aq)
Q61: Which of the following mixtures gives a