Multiple Choice
The correct result (indicating the proper number of significant figures) of the following calculation of the molecular mass for 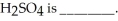 4 × 15.9994 + 32.066 + 2 × 1.0079
4 × 15.9994 + 32.066 + 2 × 1.0079
A) 98.08
B) 98.079
C) 98.074
D) 98.838
E) 98.84
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q144: 3.337 g/cm<sup>3 </sup>= _ kg/m<sup>3</sup><br>A)3.337 × 10<sup>-9</sup><br>B)3.337
Q145: What decimal power does the abbreviation d
Q146: Round the number 0.00637 to two significant
Q147: There are _ significant figures in the
Q148: The density (in g/cm<sup>3</sup>)of a gold nugget
Q150: In the following list,only _ is not
Q151: Accuracy refers to _.<br>A)how close a measured
Q152: The correct answer (reported to the proper
Q153: Mass and volume are often referred to
Q154: _ significant figures should be retained in