Multiple Choice
An atom of the most common isotope of gold, 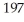 Au,has ________ protons,________ neutrons,and ________ electrons.
Au,has ________ protons,________ neutrons,and ________ electrons.
A) 197, 79, 118
B) 118, 79, 39
C) 79, 197, 197
D) 79, 118, 118
E) 79, 118, 79
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q124: Which element forms an ion with the
Q125: Which of the following elements is a
Q126: In the periodic table,the elements touching the
Q127: The correct name for Na<sub>3</sub>N is sodium
Q128: A molecule of water contains hydrogen and
Q130: What is the correct formula for ammonium
Q131: An atom of 13<sub>C contains _ protons.</sub><br>A)6<br>B)19<br>C)7<br>D)9<br>E)13
Q132: The correct name of the compound Na<sub>3</sub>N
Q133: The element calcium is in a group
Q134: How many total electrons are in the