Multiple Choice
In the symbol below,x = ________. 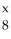 O
O
A) 17
B) 8
C) 6
D) 7
E) not enough information to determine
Correct Answer:

Verified
Correct Answer:
Verified
Q212: Which species has 18 electrons?<br>A)(<sup>39</sup>K)<br>B)(<sup>32</sup>S<sup>2-</sup>)<br>C)(<sup>35</sup>Cl)<br>D)(<sup>27</sup>Al<sup>3+</sup>)<br>E)(<sup>45</sup>Sc<sup>3+</sup>)
Q213: 200 pm is the same as _
Q214: Vanadium has two naturally occurring isotopes,<sup>50</sup>V with
Q215: Elements _ exhibit similar physical and chemical
Q216: Copper and chlorine form an ionic compound
Q218: Aluminum reacts with a certain nonmetallic element
Q219: Gravitational forces act between objects in proportion
Q220: In the Rutherford nuclear-atom model,_.<br>A)the heavy subatomic
Q221: What is the molecular formula for butane?<br>A)C<sub>2</sub>H<sub>8</sub><br>B)C<sub>3</sub>H<sub>6</sub><br>C)C<sub>3</sub>H<sub>8</sub><br>D)C<sub>4</sub>H<sub>8</sub><br>E)C<sub>4</sub>H<sub>10</sub>
Q222: Of the following,the subatomic particle with the