Multiple Choice
Silver has two naturally occurring isotopes with the following isotopic masses: 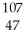 Ar
Ar 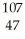 Ar 106.90509 108.9047
Ar 106.90509 108.9047
The average atomic mass of silver is 107.8682 amu.The fractional abundance of the lighter of the two isotopes is ________.
A) 0.24221
B) 0.48168
C) 0.51835
D) 0.75783
E) 0.90474
Correct Answer:

Verified
Correct Answer:
Verified
Q192: The ions Ca<sup>2+</sup> and PO<sub>4</sub><sup>3-</sup> form a
Q193: An empirical formula always indicates _.<br>A)which atoms
Q194: What is the molecular formula for propane?<br>A)C<sub>2</sub>H<sub>8</sub><br>B)C<sub>3</sub>H<sub>6</sub><br>C)C<sub>3</sub>H<sub>8</sub><br>D)C<sub>4</sub>H<sub>8</sub><br>E)C<sub>4</sub>H<sub>1</sub><sub>0</sub>
Q195: The correct name for H<sub>2</sub>SO<sub>4 </sub>is _.<br>A)sulfuric
Q196: Which atom has the largest number of
Q198: The subatomic particles located in the nucleus
Q199: Which pair of elements would you expect
Q200: What is the correct name for the
Q201: The correct formula for molybdenum (IV)hypochlorite is
Q202: Which atom has the smallest number of