Multiple Choice
The element X has three naturally occurring isotopes.The masses (amu) and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 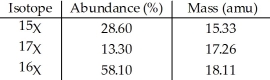
A) 17.20
B) 16.90
C) 17.65
D) 17.11
E) 16. 90
Correct Answer:

Verified
Correct Answer:
Verified
Q70: Which element forms an ion with the
Q71: The correct name for CCl<sub>4</sub> is _.<br>A)carbon
Q72: The name of the ionic compound V<sub>2</sub>O<sub>3
Q73: Which pair of elements would you expect
Q74: Elements in Group 1A are known as
Q76: Horizontal rows of the periodic table are
Q77: Different isotopes of a particular element contain
Q78: The element lithium is in a group
Q79: Which formula/name pair is incorrect?<br>A)Mn(NO<sub>2</sub>)<sub>2</sub> manganese(II)nitrite<br>B)Mg(NO<sub>3</sub>)<sub>2</sub> magnesium
Q80: The correct name for HBrO<sub>2</sub> is _.<br>A)hydrobromic