Multiple Choice
An unknown element is found to have three naturally occurring isotopes with atomic masses of 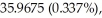
 and
and 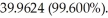 Which of the following is the unknown element?
Which of the following is the unknown element?
A) Ar
B) K
C) Cl
D) Ca
E) None of the above could be the unknown element.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Which species below is the chromate ion?<br>A)Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup><br>B)CrO<sub>4</sub><sup>2-</sup><br>C)CH<sub>3</sub>COO<sup>-</sup><br>D)CO<sub>3</sub><sup>2-</sup><br>E)None
Q18: In the symbol below,x is _. <img
Q19: Which formula/name pair is incorrect?<br>A)FeSO<sub>4</sub> iron(II)sulfate<br>B)Fe<sub>2</sub>(SO<sub>3</sub>)<sub>3</sub> iron(III)sulfite<br>C)FeS
Q20: The correct name for HClO<sub>3 </sub>is _.<br>A)hydrochloric
Q21: When calcium reacts with sulfur the compound
Q23: Which combination of protons,neutrons,and electrons is correct
Q24: 87 pm is the same as _
Q25: Which pair of atoms constitutes a pair
Q26: Potassium forms an ion with a charge
Q27: Which one of the following polyatomic ions