Multiple Choice
Element M reacts with fluorine to form an ionic compound with the formula M 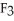 .The M-ion has 21 electrons.Element M is ________.
.The M-ion has 21 electrons.Element M is ________.
A) Al
B) Cr
C) Mn
D) Fe
E) Sc
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of the following compounds would you
Q2: What is the molecular formula for 1-hexanol?<br>A)C<sub>6</sub>H<sub>13</sub>O<br>B)C<sub>6</sub>H<sub>14</sub>O<br>C)C<sub>6</sub>H<sub>15</sub>O<br>D)C<sub>7</sub>H<sub>14</sub>O<br>E)C<sub>7</sub>H<sub>15</sub>O
Q3: Calcium is a _ and silver is
Q4: The formula of the chromate ion is
Q5: The empirical formula of a compound with
Q7: What is the molecular formula for heptane?<br>A)C<sub>6</sub>H<sub>12</sub><br>B)C<sub>6</sub>H<sub>14</sub><br>C)C<sub>7</sub>H<sub>14</sub><br>D)C<sub>7</sub>H<sub>16</sub><br>E)C<sub>7</sub>H<sub>18</sub>
Q8: The following hypothetical element : <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q9: When a metal and a nonmetal react,the
Q10: The formula of the carbonate ion is
Q11: Which one of the following basic forces