Multiple Choice
How many moles of magnesium oxide are produced by the reaction of 1.82 g of magnesium nitride with 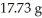 of water?
of water?
Mg3N2 + 3H2O → 2NH3 + 3MgO
A) 0.0180
B) 0.0541
C) 0.00601
D) 0.984
E) 3.00
Correct Answer:

Verified
Correct Answer:
Verified
Q91: When the following equation is balanced,the coefficient
Q92: A sample of CH<sub>4</sub>O with a mass
Q93: Lithium and nitrogen react to produce lithium
Q94: A 1.36-g sample of magnesium nitrate,Mg(NO<sub>3</sub>)<sub>2</sub>,contains _
Q95: The reaction used to inflate automobile airbags
Q97: What is the mass % of aluminum
Q98: Automotive air bags inflate when sodium azide
Q99: The molecular weight of ethanol (C<sub>2</sub>H<sub>5</sub>OH),rounded to
Q100: The molecular weight of maltose ( C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>
Q101: How many oxygen atoms are contained in