Multiple Choice
What is the empirical formula of a compound that is 66.6% C,11.2% H,and 22.2% O by mass?
A) 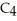
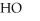
B) 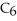
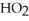
C) 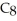
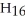
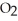
D) 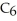
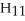
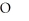
E) 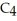
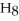
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q163: What is the mass in grams of
Q164: There are _ atoms of oxygen in
Q165: Pentacarbonyliron (Fe(CO)<sub>5</sub>)reacts with phosphorous trifluoride (PF<sub>3</sub>)and hydrogen,releasing
Q166: What is the coefficient of O<sub>2 </sub>when
Q167: The mass of a single atom of
Q169: How many moles of carbon monoxide are
Q170: What is the formula weight of lead
Q171: The molecular weight of the acetic acid
Q172: The balanced equation for the decomposition of
Q173: Magnesium burns in air with a dazzling