Multiple Choice
The balanced net ionic equation for the precipitation of calcium carbonate when aqueous solutions of Na2CO3 and CaCl2 are mixed is ________.
A) 2Na+ (aq) + CO32- (aq) → Na2CO3 (aq)
B) 2Na+ (aq) + 2Cl- (aq) → 2NaCl (aq)
C) Na+ (aq) + Cl- (aq) → NaCl (aq)
D) Ca+(aq) + CO32- (aq) → CaCO3 (s)
E) Na2CO3 (aq) + Ca 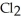 (aq) → 2NaCl (aq) + CaCO3 (s)
(aq) → 2NaCl (aq) + CaCO3 (s)
Correct Answer:

Verified
Correct Answer:
Verified
Q114: The balanced reaction between aqueous potassium hydroxide
Q115: Of the following elements,_ is the least
Q116: The molarity of an aqueous solution containing
Q117: An aliquot (28.7 mL)of a KOH solution
Q118: Which of these metals is the least
Q120: Which of the following is an oxidation-reduction
Q121: What are the respective concentrations (M)of Fe<sup>3+</sup>
Q122: The spectator ions in the reaction between
Q123: The concentration of iodide ions in a
Q124: Which combination will produce a precipitate?<br>A)NaC<sub>2</sub>H<sub>3</sub>O<sub>2 </sub>(aq)and