Multiple Choice
The molarity (M) of an aqueous solution containing 22.5 g of sucrose (C12H22O11) in 35.5 mL of solution is ________.
A) 0.0657
B) 1.85 × 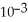
C) 1.85
D) 2.33
E) 0.634
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q155: Calculate the number of grams of unknown
Q156: What is the concentration (M)of a NaCl
Q157: All of the following are true concerning
Q158: The molarity (M)of an aqueous solution containing
Q159: What is the concentration of nitrate ions
Q161: What volume (mL)of a concentrated solution of
Q162: In which species does nitrogen have the
Q163: How many grams of hydrazine N<sub>2</sub>H<sub>4</sub> must
Q164: A one-hundredfold dilution of an enzymatic buffer
Q165: Which solution has the same number of