Multiple Choice
What volume (mL) of a concentrated solution of sodium hydroxide (6.00 M) must be diluted to 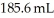 to make a
to make a 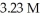 solution of sodium hydroxide?
solution of sodium hydroxide?
A) 99.9
B) 3600
C) 287
D) 0.104
E) 0.0100
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: The net ionic equation for the reaction
Q86: A 650 mL sodium bromide solution has
Q87: How many milliliters of a 14.2 M
Q88: The molarity of a solution prepared by
Q89: Which of the following would require the
Q91: The point in a titration at which
Q92: The process by which metal in the
Q93: Sodium does not occur in nature as
Q94: The spectator ions in the reaction between
Q95: When 0.344 mol of HCl is combined