Multiple Choice
What volume (mL) of 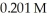 potassium hydroxide will it take to reach the equivalence point in a 16.3 mL aliquot of
potassium hydroxide will it take to reach the equivalence point in a 16.3 mL aliquot of 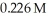 triprotic acid?
triprotic acid?
A) 2.22
B) 18.3
C) 55.0
D) 6.11
E) 120
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q133: The net ionic equation for formation of
Q134: The compound NH<sub>4</sub>Cl is a weak acid.
Q135: If it took 49.33 mL of 0.2454
Q136: Which of the following is insoluble in
Q137: Aqueous solutions of a compound did not
Q139: Aqueous potassium chloride will react with which
Q140: The compound HClO<sub>4</sub> is a weak acid.
Q141: A weak electrolyte exists predominantly as _
Q142: How much CaF<sub>2</sub> (g)is formed when <img
Q143: Silver ions can be precipitated from aqueous