Multiple Choice
A titration reached the equivalence point when 16.1 mL of 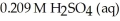 was added to
was added to 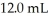 of NaOH (aq) of unknown concentration.What is the concentration (M) of this unknown NaOH solution?
of NaOH (aq) of unknown concentration.What is the concentration (M) of this unknown NaOH solution?
A) 0.561
B) 80.8
C) 0.280
D) 0.140
E) 3.21
Correct Answer:

Verified
Correct Answer:
Verified
Q3: What is the concentration (M)of 39.88 mL
Q4: Mixing 1.00 mL of an aqueous solution
Q5: The solvent in an aqueous solution is
Q6: The net ionic equation for formation of
Q7: What volume (mL)of 7.48 × 10<sup>-2</sup> M
Q9: How many moles of CaCl<sub>2</sub> are formed
Q10: How many moles of Na<sup>+</sup> are present
Q11: How many grams of H<sub>3</sub>PO<sub>4</sub> are in
Q12: Which hydroxides are weak bases?<br>A)KOH<br>B)Ba(OH)<sub>2</sub><br>C)RbOH<br>D)CsOH<br>E)None of these
Q13: What are the spectator ions in the