Multiple Choice
The value of ΔH° for the reaction below is -6535 kJ. ________ kJ of heat are released in the combustion of 16.0 g of 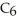
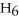 (l) ?
(l) ?
2C6H6 (l) + 15O2 (g) → 12CO2 (g) + 6H2O (l)
A) 1.34 × 103
B) 5.23 × 104
C) 669
D) 2.68 × 103
E) -6535
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: The _ of a reaction is the
Q34: What is the molar heat capacity (in
Q35: The ΔH<sub>vap </sub>of water is 40.7 kJ
Q36: The value of ΔH° for the reaction
Q37: The molar heat capacity of an unknown
Q39: The primary component of natural gas is
Q40: _ is defined as the energy used
Q41: All of the following are considered fossil
Q42: Given the following reactions 2S (s)+ 3O<sub>2</sub>
Q43: Given the following reactions N<sub>2</sub> (g)+ 2O<sub>2</sub>