Multiple Choice
The value of ΔH° for the reaction below is -1107 kJ: 2Ba (s) + O2 (g) → 2BaO (s)
How many kJ of heat are released when 5.75 g of Ba (s) reacts completely with oxygen to form 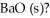
A) 96.3 kJ
B) 26.3 kJ
C) 46.4 kJ
D) 23.2 kJ
E) 193 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: The units of heat capacity are _.<br>A)K/J
Q19: The ΔE of a system that absorbs
Q20: A sample of calcium carbonate [CaCO<sub>3</sub> (s)]
Q21: When a system _,ΔE is always negative.<br>A)absorbs
Q22: The value of ΔH° for the reaction
Q24: A chemical reaction that releases heat to
Q25: Which one of the following is an
Q26: The kinetic energy of a 7.3 kg
Q27: The value of ΔH° for the reaction
Q28: The change in the internal energy of