Multiple Choice
Given the data in the table below,ΔH°rxn for the reaction 3NO2 (g) + H2O (l) → 2HNO3 (aq) + NO (g)
Is ________ kJ. 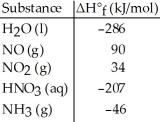
A) 64
B) 140
C) -140
D) -508
E) -64
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q104: A 23.2 g piece of space debris
Q105: Given the following reactions N<sub>2</sub> (g)+ O<sub>2</sub>
Q106: A chemical reaction that absorbs heat from
Q107: The energy released by combustion of _
Q108: Calculate the kinetic energy in joules of
Q110: In the reaction below,ΔH°<sub>f</sub> is zero for
Q111: For a given process at constant pressure,w
Q112: The specific heat capacity of liquid mercury
Q113: How much heat is released when 29.5
Q114: When 0.800 grams of NaOH is dissolved