Multiple Choice
Given the data in the table below,ΔH°rxn for the reaction 2Ag2S (s) + O2 (g) → 2Ag2O (s) + 2S (s)
Is ________ kJ. 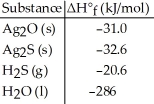
A) -1.6
B) +1.6
C) -3.2
D) +3.2
E) The ΔH°f of S (s) and of O2 (g) are needed for the calculation.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q78: The reaction 4Al (s)+ 3O<sub>2</sub> (g)→ 2
Q79: Of the following,ΔH°<sub>f</sub> is not zero for
Q80: Given the following reactions 2NO → N<sub>2</sub>
Q81: What is the kinetic energy of a
Q82: An 6.11 g sample of calcium carbonate
Q84: Calculate the kinetic energy in joules of
Q85: The value of ΔH° for the following
Q86: All of the following statements are true
Q87: For the combustion reaction of methane,ΔH°<sub>f</sub> is
Q88: The ΔE of a system that releases